To add a biobank or a patient registry to the system, click on the "Propose" button marked by the red arrow.
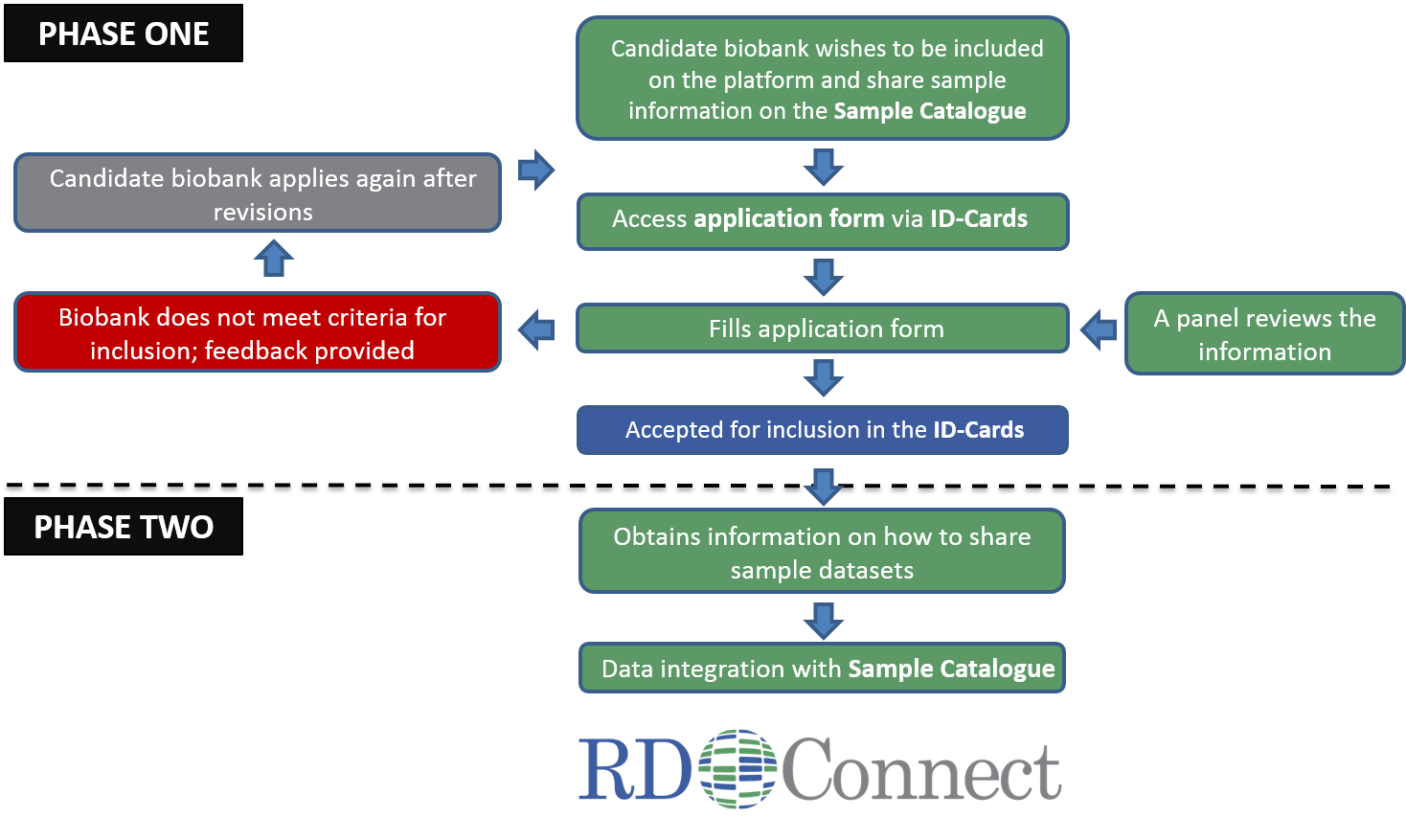
To share biosample collections in the RD-Connect Sample Catalogue, the applying biobank first needs to fill the application form via the Registry & Biobank Finder. The application in reviewed by the Panel for Biobank Assessment. If the opinion is negative, the panel gives feedback to the biobank, which is encouraged to make the suggested improvements and apply again. The biobanks that receive approval from the Panel receive information how to upload information about their sample collections in the Sample Catalogue.
How to:
![]() Registry & Biobank Finder - user guide for registry and biobank managers
Registry & Biobank Finder - user guide for registry and biobank managers
![]() Sample Catalogue - user guide for biobank managers
Sample Catalogue - user guide for biobank managers
![]() Sample Catalogue - browsing sample data (slides from the webinar for biobank managers)
Sample Catalogue - browsing sample data (slides from the webinar for biobank managers)
![]() Sample Catalogue - uploading sample data (slides from the webinar for biobank managers)
Sample Catalogue - uploading sample data (slides from the webinar for biobank managers)
![]() Sample Catalogue - template for sample upload
Sample Catalogue - template for sample upload
Assessment criteria
1. Disease(s) of interest: Interested biobanks must already store collections of rare disease biological samples; the RD-Connect Panel for Biobank Assessment will evaluate the candidate biobank on the basis of the rare disease focus of the sample collection, which must be clearly described.
2. Quality Standards (QS): Biobanks should have a quality system in place for the operational management, including quality assessment/quality control for sample and data management. QS for sample management should follow best practice guidelines such as those of the OECD or, in the future, the upcoming European standards for pre-analytical treatment of biological samples.
3. Standard Operating Procedures (SOPs): The biobank should have already adopted SOPs regulating:
- sample (and data) acquisition
- testing to ensure sample (and data) integrity
- sample processing and storage.
4. Ethical, Legal and Social Implications (ELSI) The biobank should adhere to the following ELSI principles:
- samples are collected only if an appropriate informed consent has been previously obtained
- the consent can be withdrawn and sample (and data) removed anytime
- confidentiality issues need to be dealt with in compliance with the local government laws
- specific rules and policies for distributing samples (and data) are clearly defined
5. Informatics set-up: The biobank should meet the following minimum system requirements:
- centralised or distributed data repository with internet access
- information Management system to manage biobank repositories, resources, samples and data in a structured way
- data identification capabilities (local persistent unique identifiers for samples and sample data)
- data transfer capabilities (exportability of data from the management system to a file format and secure data transfer through the Internet)